The Chemist’s Dilemma: Plastics Help Some But Harm Others
Matthew HartingsEnvironmentally persistent plastics, and the chemical additives associated with them, pose a challenge to ecological and public health. The plastic consumer goods that end up as litter directly affect marine life and the food webs that rely on clean water. Additionally, the ubiquity of chemical additives in plastics, which are endocrine disruptors, has led to concern about their potential influence on human health. The chemical imprint of plastics in the environment is inescapable.
For humans, there are two primary routes by which plastic waste can affect health. Plastic particles, formed from the erosion of larger objects, can damage cells and tissue by inducing oxidative stress and inflammation.(1) Second, the chemicals that are added to plastics to facilitate their durability and industrial processing can cause liver toxicity and disruption of endocrine cycles.(2) Additionally, ocean litter can pose a disproportionate threat to the public health and economies of small islands and ocean or sea-bordering developing states.(3)
Consider the straw
The recently newsworthy plastic straw is both a stark representation of the success of modern chemistry and a symbol of the clarion call demanding that the field of chemistry must fundamentally change to meet our needs for durable plastics that won’t cause harm, particularly when they’re discarded.
The state of art behind the production of plastic straws (and most of our other plastic consumer goods) is part science and part serendipity. The foundation of plastics starts with molecules called polymers. Polymers are long chains of repeating chemical parts. To be a good plastic, a group of polymers must be able to melt, be molded into an interesting shape, and be able hold that shape while it sets. There are other basic properties that are important, too, such as being sturdy and stable. A straw, for instance, is flexible, while a rigid Lego brick will inflict searing pain on a bare foot. In either case, the plastic doesn’t crack or crumble.
It is not often that a polymer, on its own, has all of the properties that are demanded of it by an end-use application. These cases are often where some serendipity come into play. Special additives can be used to fine tune the characteristics of the plastic. Some guesswork is involved in identifying just the right additive to give the product just the right property. This process could be informed by a view to the population health impacts of choices made.
Durability: a double-edged sword
In the past, some of the most important work done by polymer chemists has gone into improving plastic durability. Straws need to be free of cracks. Plastic food storage containers need to hold up after multiple uses and multiple washings. A Lego brick must be able to stab a bare foot for years after it was first used.
Unfortunately, the lasting power of plastics is the main reason they are so difficult to remove from the environment. Single-use plastic straws and beverage containers aren’t always recycled. Even when they are disposed of properly, they will often fall out of the waste stream when refuse is transferred from one container to another, contaminating the environment.
One step that many environmental advocates are endorsing is the outright banning of plastic straws from restaurants, and some cities have complied. Unfortunately, this action will have unintended effects on people with disabilities who rely on the functionality provided by single-use straws.
BPA: friend and foe
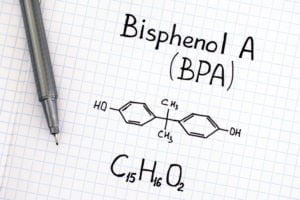 Another plastic target of many public health advocates is Bisphenol A (BPA), referred to by polymer scientists as a plasticizer. BPA has been cited as an endocrine disruptor, capable of altering animal hormone pathways. While the US Food and Drug Administration’s most thorough studies have shown that typical exposure levels to BPA are not dangerous(4), there are many independent academic scientists whose experiments show cause for concern.(5)
Another plastic target of many public health advocates is Bisphenol A (BPA), referred to by polymer scientists as a plasticizer. BPA has been cited as an endocrine disruptor, capable of altering animal hormone pathways. While the US Food and Drug Administration’s most thorough studies have shown that typical exposure levels to BPA are not dangerous(4), there are many independent academic scientists whose experiments show cause for concern.(5)
BPA is ubiquitous in plastic products because it is so good at making polymers behave in production the way industrial engineers need them to, and BPA also helps keep the plastics more durable during use. This means that plastics are more resistant to damage, and the consumer goods they comprise will not fail. So removing BPA and similar chemicals from the production stream could reduce risk of exposure to this additive, but comes at a cost to quality of life and public health. The ease of processing polymers with BPA reduces the price of consumer goods, and using BPA-containing plastics in food storage containers prolongs shelf life and increases access to nutritious fruits and vegetables.
In short, the durability of both the plastics and BPA in consumer goods render them difficult to remove from the environment where they can harm humans and wildlife. However, their utility, particularly to certain groups, often deems plastics necessary to support both human health and quality of life. For example, people experiencing homelessness may rely on bottled water and packaged food, people with disabilities often require straws, and many groups need medical equipment (prosthetics, IV tubes) made with plastics.
Can we find a kill switch?
The challenge for chemists, then, is to develop plastics and plasticizers that have their own shelf life. Can chemicals be engineered with a kill switch—something that will cause them to degrade into less harmful and impactful materials when they’re no longer needed? Nature has employed this type of polymer degradation for millennia. Enzymes such as proteases convert biological polymers such as proteins into individual amino acids and other necessary nutrients. The goal for chemists should be to replicate this type of biological machinery for industrial plastics and plasticizers.
 This is no small task. Designing and choosing the chemistry to control polymer and plasticizer degradation will be difficult on its own. Choosing the end products of plastic breakdown is another challenge altogether, and one that will need to include input from biological and environmental ethicists, public health professionals, and ecologists. This process will add a new source of chemicals into our environment and, as such, will require that these chemicals be benign. Fifty years ago, we might have chosen carbon dioxide as our choice for an ideal end product. However, the effects of global warming from greenhouse gases now highlight that there is no such thing as a completely harmless chemical.
This is no small task. Designing and choosing the chemistry to control polymer and plasticizer degradation will be difficult on its own. Choosing the end products of plastic breakdown is another challenge altogether, and one that will need to include input from biological and environmental ethicists, public health professionals, and ecologists. This process will add a new source of chemicals into our environment and, as such, will require that these chemicals be benign. Fifty years ago, we might have chosen carbon dioxide as our choice for an ideal end product. However, the effects of global warming from greenhouse gases now highlight that there is no such thing as a completely harmless chemical.
This task seems very daunting. However, scientists have never been known to shrink away from a challenge. Using the counsel and direction given from experts outside of their field, chemists can shift their focus from designing durable materials to designing materials that serve their intended roles (medical equipment, safe storage of food, Lego brick foot torture) for only as long as they are needed. They can also work with colleagues from the population health sciences who are mindful of the possible health hazards, but also the ways that plastics enable people to obtain fresh foods, cutting edge medical devices, and other things positive for health.
References
(1) https://pubs.acs.org/doi/pdfplus/10.1021/acs.est.6b02569
(2) https://link.springer.com/chapter/10.1007/978-3-319-16510-3_5
(3) http://havsmiljoinstitutet.se/digitalAssets/1641/1641020_sime-2017-4-marine-plastic-litter.pdf
(4) https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm598100.htm
(5) http://www.sciencemag.org/news/2017/02/bpa-safety-war-battle-over-evidence
https://www.newsweek.com/bpa-safe-fda-touts-study-minimal-effects-experts-wary-818833






All comments will be reviewed and posted if substantive and of general interest to IAPHS readers.